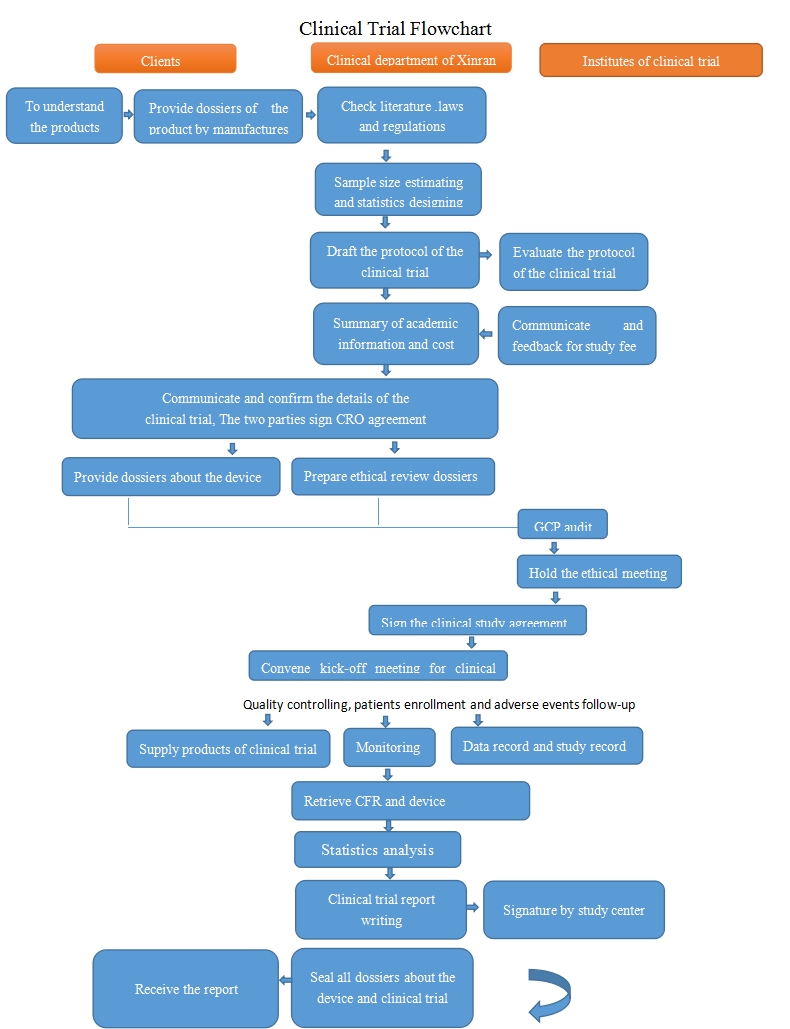
Clinical trial is the most crucial step of medical device registration process with long operation period and high cost .The systematic and professional process shall comply with regulations as well as the ethics and scientific principles.
Based on long-term cooperation with the clinical trial institutes and experts; with the professional team led by legal and medical experts and real-time laws and regulations follow-up, the scientific protocol design, systemic management and quality control ensures that the clinical trial process comply with ICH-GCP and China's GCP requirements, with the aim of reducing research risk, research funding, and promoting the process of market approval. We provide the following service:
1. Pre-study research
2. Drawing up the study protocol
3. Visiting and screening Clinical institutes
4. Getting Ethnics review approval
5. Kick-off meeting for clinical trial
6. Clinical trial monitoring and inspecting
7. Clinical data management
8. Statistical analysis of clinical data
9. Clinical trial report writing
10. Sign and seal clinical trial report